Marco Verità, Laboratorio di Analisi dei Materiali Antichi LAMA, Università IUAV di Venezia, Venezia (Italy)
Venetian innovations in glassmaking and their influence on the European glass history
Glass composition through the analyses
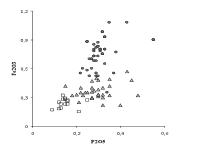
Although it has remained a "secret" up to present times, the chemical analysis of a significant number of Venetian glass samples has revealed the procedure adopted to stabilize crystal glass and suggests which solution was used by the glassmakers to give to the cristallo an acceptable durability. The chemical analysis of Venetian clear glass of the sixteenth and seventeenth centuries allowed a group of glasses to be identified, whose CaO, MgO and P2O5 content is lower than that of common and vitrum blanchum glass (the common glass samples were distinguished for their slight natural colour from the colourless samples) (Verità 1985). In these glasses the iron content is also much lower than in the two other groups. All these elements in lower concentration were introduced into the glass with the ash, in which they are present as salts that are insoluble in water. The composition of these glasses, therefore, is compatible with the purification process of soda ash described above for the preparation of crystal glass.
In Table 1 the mean composition, standard deviation, minimum and maximum values of the three groups of Venetian glass are reported. Part of the analyses was previously published in (Verità and Zecchin 2009). The analyses refer to sixteenth-seventeenth century samples, and concern more common samples (58) than vitrum blanchum (32) and cristallo (15) samples. Traces of lead and tin were detected in all the common glass samples and in three of the vitrum blanchum samples, while no traces were found in the cristallo samples. Lead and tin were added in high concentration to the transparent glass to make lattimo, the white opaque glass of the Venetian glassmakers. Small amounts of these elements could be present in the glass cullet and transfer to the molten glass. These results show that in order to minimize any contamination and ensure a high quality for cristallo, cullet was excluded from the batch, whereas it was used for common glass.
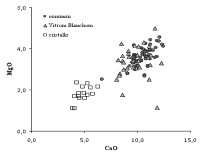
Fig. 1. Diagram of magnesium versus calcium oxides for the analysed samples. Symbols indicate type of glass, as in the figure.
In the following diagrams, some of the most important components of Venetian glass are discussed using the data summarized in Table 1. Different symbols for the three types of glass were used. In Fig. 1, the concentrations of calcium and magnesium oxides are reported. These concentrations are lower in cristallo than in common and vitrum blanchum glass (the average values are about one half, see Table 1), but not near zero. This indicates that not only purified soda ash was used as a flux, and that some other ingredient was added by the glassmakers to stabilize cristallo. The glass technology of that time ignored the use of lime and magnesia to make the glass resistant to weathering. These oxides were introduced accidentally into the composition in the form of plant ash components. The assumption that the Venetian glassmakers had understood the importance of lime to improve glass durability, and that they first started the transition from a two-component to a three-component glass batch (as in modern glass), is not supported by any written evidence of the purchase or use of Ca-bearing ingredients (marble, calcareous materials, dolomite) for glassmaking in Venice in this period. At the moment, it is not known exactly where and when the voluntary addition of a source of lime became a common practice (probably in Bohemia during the seventeenth century). The most readily available and controllable source of lime and magnesia was vitrum blanchum glass frit (or cullet). The glassmakers might have melted a batch made of cristallo and vitrum blanchum frit (probably in equal amounts). This process would cause a moderate loss in transparency and clarity, still yielding a product of higher quality than vitrum blanchum, but it would ensure a sufficient amount of calcium and magnesium to stabilize the glass.
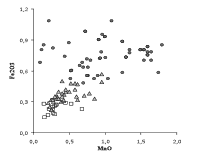
Other elements whose concentration is lower in cristallo as a result of the purification of soda ash are iron and phosphorous (Fig. 2). The beneficial effect of the use of purified soda ash on the optical properties of glass is shown in Fig. 3. A clear discontinuity in the iron content can be observed between common glass and the other two types of glass. To the lower iron content corresponds a lower addition of manganese in particular to the crystal glass. This leads to a less grey and more transparent glass. The vitrum blanchum shows an intermediate iron content between common glass (Fe2O3 between 0.7 and 1.0%, with very variable values of manganese), and cristallo (Fe2O3 0.2-0.3%). A decrease of iron content in the Venetian glass of the sixteenth and seventeenth centuries as compared to the previous centuries is evident not only for cristallo. The average content of Fe2O3 shifts from 0.60% of the fifteenth century samples to 0.45% of the seventeenth century glass. These values and the low standard deviation of the iron analyses (less than 0.1%) point out the special care taken by the Venetian glassmakers in reducing colouring contaminants by means of a careful choice of raw materials, the selection of cullet and care in the use of metallic tools.
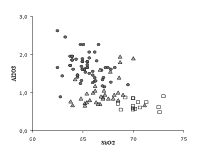
Beside the purification of the plant ash, other improvements of the glass quality of the Venetian glass emerge from the analyses. The alumina content (Fig. 4) is lower and the silica content is higher in the high quality Venetian glass as compared to common glass. These data confirm the skill of the Venetian glassmakers in selecting and using less contaminated sources of quartz. Finally, the results in Table 1 show an increase of the averaged content of K2O compared with the analyses of the previous centuries (the mean values of K2O are on the contrary very constant, near 3%!). The voluntary addition of this oxide in the form of calcined tartar (a potash-bearer) to the soda-lime glass was ascertained in the recipes of the Venetian glassmakers of the fifteenth to the seventeenth centuries (Zecchin P., 1997). The reasons for these additions are not clear, maybe they were meant to improve the optical properties of the glass (a higher refraction index leading to a brighter glass).
At the end of this short discourse on in the Venetian glass innovations, it is interesting to recall an important role that this glass played in the history of science. Galileo Galilei made the first "occhiali da veder lontano" (telescope lenses) using selected Muranese mirror glass. Galileo complained about the defects of the Venetian glass, probably the same we have seen before. In order to get higher quality glass, in 1610 Galileo succeeded in persuading the Granduca of Tuscany to expressly make in Florence a furnace to produce glass for lenses. But this experiment was unsuccessful, and few years later Galileo was compelled to turn to the Muranese glassmakers again for the glass lenses for his telescopes (Verità, 2008).